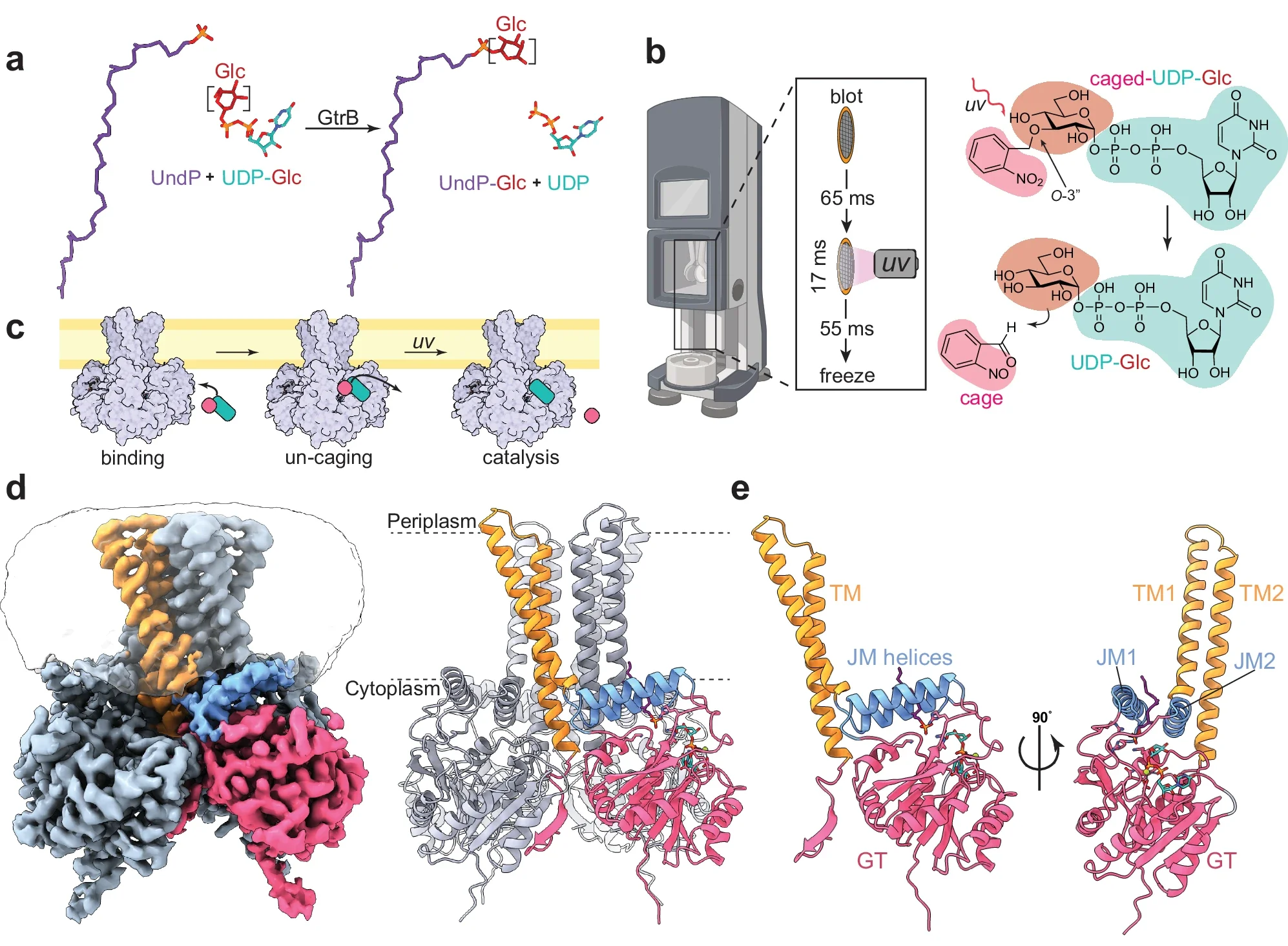
(a) Reaction catalyzed by GtrB. The Glc moiety (maroon) on UDP-Glc (teal) is transferred onto the UndP (purple) headgroup by GtrB, forming UndP-Glc. (b) Cartoon representation of ‘Flash-and-freeze’ system for cryo-TREM showing the travel of the cryo-EM grid before being vitrified on the left. (c) Cartoon of theoretical un-caging (pink) of UDP-Glc (teal) when bound to membrane-embedded GtrB after UV irradiation resulting in catalysis. (d) Cryo-EM density map of the substrate-bound state GtrB bound to UndP and UDP-Glc. On the right, the ribbon model of the substrate-bound state is shown with approximate membrane boundaries represented as dotted lines (e) Ribbon model of a GtrB protomer.
Our Research
All proteins that are integral to the cellular membrane are endowed with the ability to reside and function in a hydrophobic environment. Over the last twenty years, mostly thanks to the dramatic improvement in our ability to express, purify and determine their atomic structure, we have learned a great deal about the principles that govern structure and activity of membrane proteins. Despite these advances, there has been little study of how integral membrane proteins interact with hydrophobic, lipidic, substrates, associations that are required for the enzymatic biosynthesis and modification of cellular membranes, and for the transport of lipids or lipidic ligands across the bilayer or from one leaflet to the other.
The principles governing recognition, specificity, and function of membrane proteins that interact with hydrophobic substrates are best investigated by high-resolution structures. An atomic level structure is unique in providing detailed insight into these processes at a molecular level, revealing the precise protein residues involved in substrate recognition, catalysis or translocation, and, overall, providing us with hypotheses, which can then be tested by functional assays and biophysical techniques. My main research interests, which often overlap are (1) to understand how the membrane bilayer and specific membrane enzymes and transporters interact to accommodate lipidic substrates and (2) to use structural biology techniques to understand the molecular bases of drug resistance.
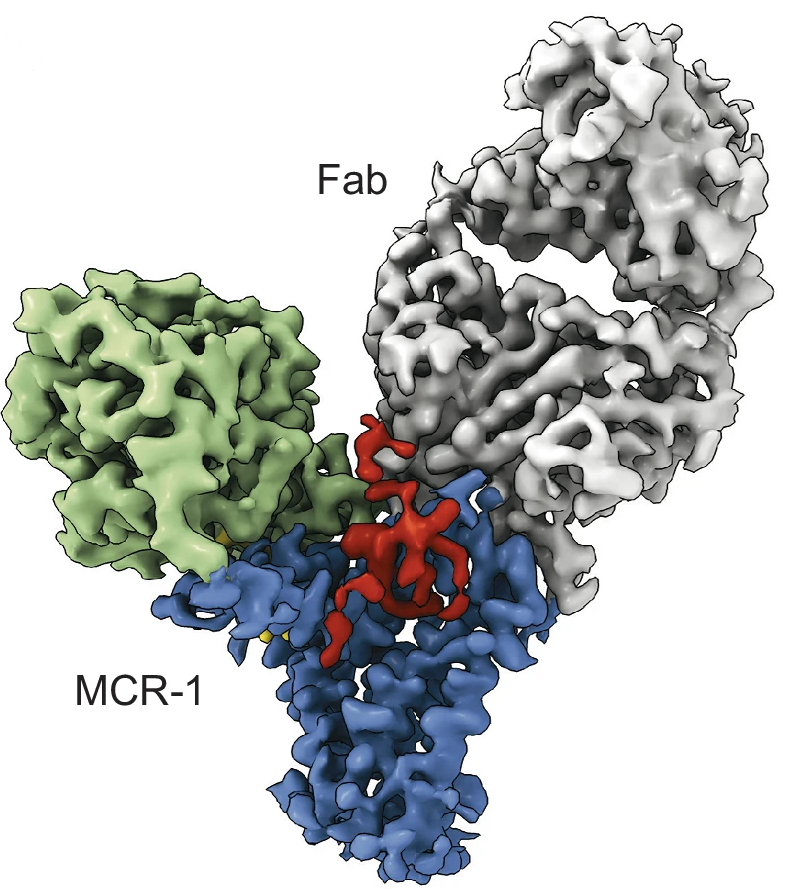
Cryo-EM density map of the PE- and KLA-bound MCR-1–Fab complex. Density corresponding to the Fab is shown in gray, and the PD and TM domain of MCR-1 are colored green and blue, respectively. The density for KLA is in red, and the density for PE in yellow.
Structure and function of integral membrane lipid-modifying enzymes
Cellular membranes are critical components of all free-living organisms. However, knowledge of their biosynthesis and modification has been hindered by the hydrophobicity engendered by their lipid constituents. Lipids are synthesized and modified primarily by integral membrane enzymes embedded, at least in part, in the bilayer, but the atomic-level details of lipid/enzyme interactions and the determinants of their specificity remain poorly understood. To shed light on this question, we are studying the structure and function of distinct families of integral membrane lipid-modifying enzymes. For example: GtrB, a polyisoprenyl phosphate glycosyltransferase attaches glucose to a lipid carrier for membrane translocation and a glycosyl donor for subsequent reactions. This reaction represents the first step in all protein glycosylation and glycosylation of the cell wall. ArnT uses sugar-charged donors produced by GtrB-like enzymes, and transfers the saccharide to lipid A on the cell surface of bacteria, altering antibiotic resistance properties. We are exploring substrate recognition by these enzymes with a combination of experimental approaches including x-ray crystallography, cryo-EM, and recently time-resolved cryo-EM to study high-resolution enyzmatic dynamics.
The transport of lipidated Wnts by their sole specific carrier Wntless
Wnts are evolutionarily conserved ligands that signal at short range to regulate morphogenesis, cell fate and stem cell renewal. The first and essential steps in Wnt secretion are their O-palmitoleation by the enzyme PORCN and subsequent loading onto the dedicated transporter WLS/Evi. O-palmitoleated Wnts associated with WLS then travel from the ER to the plasma membrane, where they are transferred to receptors, such as Frizzled, on the membranes of target cells, in turn triggering the activation of signaling pathways.
Structure of WNT8A in complex with WLS. The 3.2 Å structure of WNT8A bound to WLS with WLS colored in rainbow from N- (blue) to C- (red) terminus, and WNT8A in violet with a transparent surface. The PAM is represented as green spheres extending out between TM helices 4 and 5. Glycosylation of WNT8A at two sites is in red, as sticks.
We determined the 3.2Å resolution cryo-EM structure of palmitoleated human WNT8A in complex with WLS its dedicated carrier, accompanied by biochemical experiments – performed by our close collaborator Dr. David Virshup at Duke-NUS in Singapore – to probe the physiological implications of the observed association (Nygaard, et al., Cell, 2021). We show, for the first time, that the WLS membrane domain has close structural homology to G protein-coupled receptors (GPCR). A Wnt hairpin inserts into a conserved hydrophobic cavity in the GPCR-like domain with the PAM protruding between two helices into the bilayer. A large opening to the bilayer within the GPCR-like membrane domain of WLS may delineate the route for how the PAM is shuttled from PORCN to WLS in an energetically favorable way. By comparing our structure to that of Wnt in complex with the binding domain of Frizzled, we noticed a large conformational change on a separate Wnt hairpin which may be the key to understating its one-way transfer to receiving cells. In summary, our work provides molecular-level insights into a central mechanism in animal body plan development and stem cell biology, and opens up a fascinating new direction for the lab to explore membrane protein – lipid interactions.
Kim, J., Tan, Y.Z, Wicht, K.J.,3, Erramilli, S.K., Dhingra, S.K, Okombo. J., Vendome, J., Hagenah, L.M., Giacometti, S.I., Warren, A.L, Nosol, K., Roepe, P.D, Potter, C.S., Carragher, B., Kossiakoff, A.A., Quick, M., Fidock D.A. & Mancia, F. (2019) Structure and drug resistance of the Plasmodium falciparum transporter PfCRT. Nature (2019).
Structure and drug resistance of the Plasmodium falciparum transporter PfCRT.
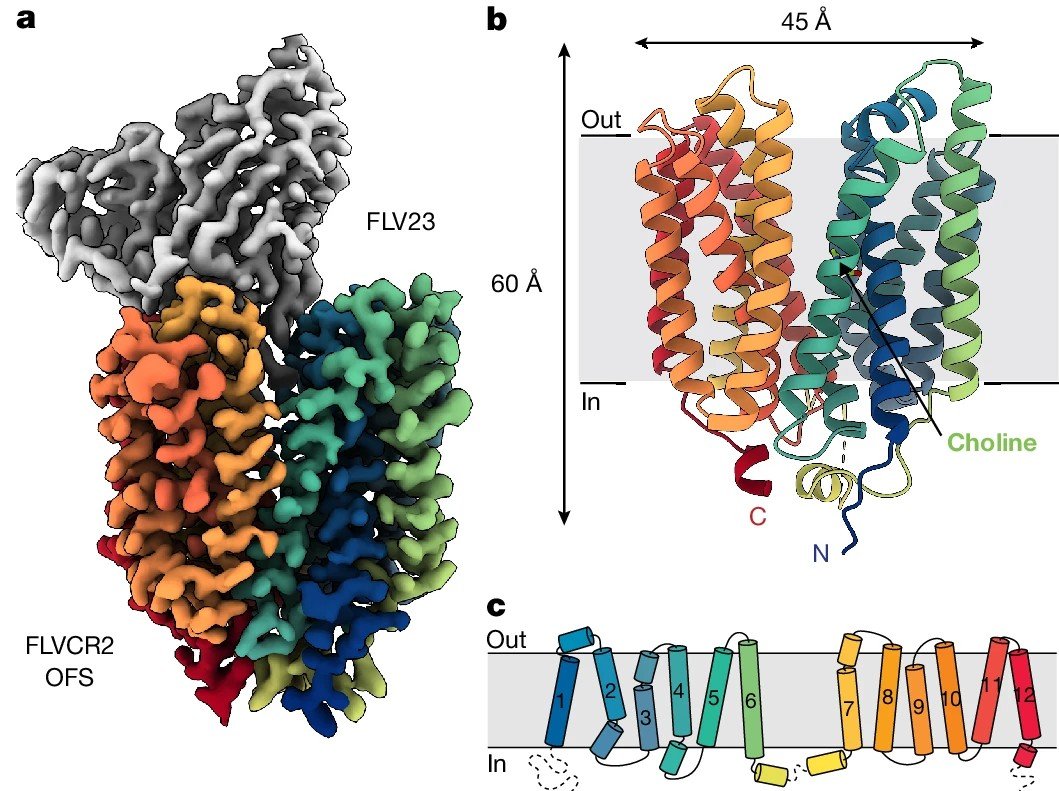
Cater, R.J., Mukherjee, D., Gil-Iturbe, E. et al. Structural and molecular basis of choline uptake into the brain by FLVCR2. Nature (2024).
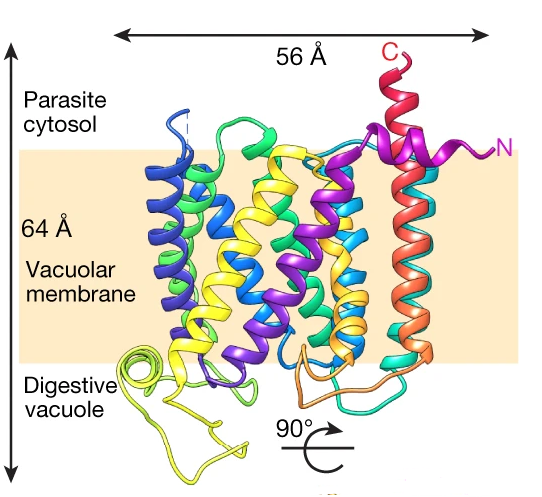
Drug resistance in Plasmodium falciparum (Pf), the deadliest of the malaria parasites that threatens almost half the world’s population, has been associated with mutations in specific genes. The protein responsible for parasite resistance to both previously and currently used first-line antimalarials, chloroquine (CQ) and piperaquine (PPQ), is the 48-kDa P. falciparum chloroquine resistance transporter (PfCRT). PfCRT resides on the DV membrane and mediates drug resistance via active drug efflux. Our progress in understanding the molecular basis of PfCRT-mediated drug resistance, has been seriously hampered by the lack of an atomic model of this transporter. Using antigen-binding fragment technology and single-particle cryo-electron microscopy (cryo-EM), we have determined the structure of a CQ-resistant isoform of PfCRT to 3.2 Å resolution. Combining structural information, with biochemistry, genetics and parasitology, we have gained insights on the molecular mechanism of PfCRT-mediated drug resistance, identified markers for the development of resistance, and set the bases for future prospects in structure-guided drug design
Structure of STRA6 receptor for retinol uptake
Vitamin A is an essential nutrient for all mammals. Many biological processes, including and foremost vision, are crucially dependent on its adequate supply for proper function. Alterations of vitamin A metabolism can result in a wide spectrum of ocular defects and lead to blindness. Retinol (vitamin A alcohol) is the predominant circulating vitamin A form in the fasting state. In times of need (i.e. in the absence of dietary vitamin A intake), in order to distribute vitamin A to the target peripheral tissues, retinol is released in the bloodstream from the liver, the main body storage site of the vitamin, bound to retinol- binding protein (RBP). Inside the cells, retinol binds specific intracellular carriers, namely cellular retinol-binding proteins, and it serves as a precursor for the active vitamin A forms: retinaldehyde, critical for vision, and retinoic acid, the ligand for specific nuclear receptors that regulate the transcription of hundreds of target genes. STRA6, the putative plasma membrane receptor for RBP, was identified in 2007. However, its mechanism of action has remained elusive, not least due to the absence of any structural information. We have determined the structure of STRA6 determined to 3.9 Å resolution by single-particle cryo-electron microscopy (improved to 3.1 Å resolution with protein reconstituted in nanodisc). The atomic model of STRA6 provides a template to guide our understanding at a molecular level on how this protein may function, and to further investigate its physiological role.
Transport of nutrients across the blood brain barrier (BBB)
Choline and Omega-3 fatty acids are essential nutrients that the human body needs in vast quantities for cell membrane synthesis, epigenetic modification and neurotransmission required to support the growth, maintenance, and function of the central nervous system (CNS). The brain has a particularly high demand for choline and omega-3, but how they enters the brain remained unknown. We demonstrated both in vivo and in vitro that FLVCR2 is a BBB choline transporter and is responsible for the majority of choline uptake into the brain. We also determined the structures of choline-bound FLVCR2 in both inward-facing and outward-facing states using cryo-electron microscopy. Additionally, omega-3 fatty acids are transported across the blood–brain and blood–retina barriers in the form of lysophosphatidylcholine by major facilitator superfamily domain containing 2A (MFSD2A) in a Na+-dependent manner. We presented the structure of MFSD2A determined using single-particle cryo-electron microscopy providing insights into the molecular mechanism by which this atypical major facility superfamily transporter mediates the uptake of lysolipids into the brain.